Custom Lentivirus
Lentifect™ Custom Lentivirus
Lentifect™ custom lentivirus production services are available for nearly any GeneCopoeia clone type, including:
To order custom Lentifect™ purified lentiviral particles
visit our
search page.
Lentiviral vectors are potent vehicles for delivery of
genes into a wide range of cell types including difficult-to-transfect cells. Lentiviral particles transduce cells
and integrate into the host genome in dividing and post-mitotic cells resulting in long-term expression of the
transgene in vitro. Producing, concentrating and titrating lentiviral particles, however, is time consuming
and requires some experience to achieve high titers and consistent results. GeneCopoeia’s Lentifect™ purified
lentiviral particles save you time and effort, and provide high-level expression of genetic elements of interest
(Figure 1).
Transduction of H1299 cells with GeneCopoeia’s Lentifect™ lentiviral particles expressing eGFP.
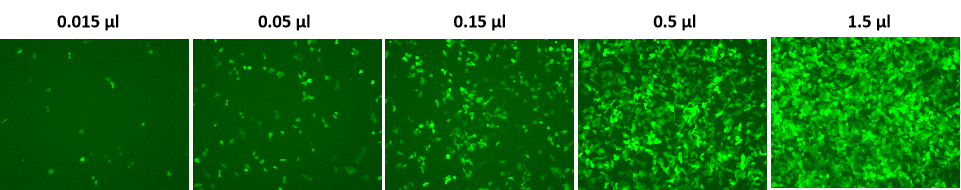
Figure 1. H1299 cells in 24-well plates were transduced with the indicated amounts of eGFP purified particles, (catalog number LP303) in the presence of 5 µg/ml of polybrene. The expression of eGFP was visualized with a fluorescence microscope 72 hours post-transduction.
Transduction of H1299 cells with GeneCopoeia’s Lentifect™ lentiviral particles expressing mCherry.
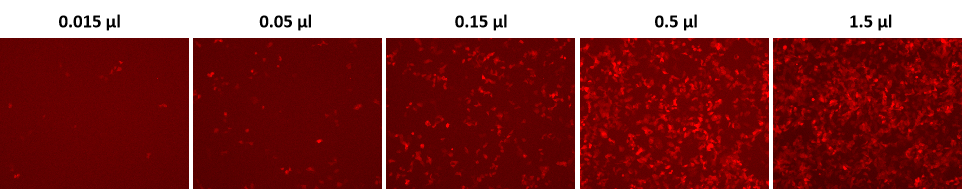
Figure 2. H1299 cells in 24-well plates were transduced with the indicated amounts of mCherry purified particles, (catalog number LP151) in the presence of 5 µg/ml of polybrene. The expression of mCherry was visualized with a fluorescence microscope 72 hours post-transduction.
FAQs
Frequently Asked Questions: Lentivirus
Answer: The best way is to
search for what you need on our lentiviral search page. You simply need to
indicate the clone type (ORF cDNA, promoter, etc.), the gene information (gene symbol, aliases, description,
nucleotide accession, Entrez gene ID, catalog or product ID), the vector type, and the delivery volume. You can
also request a custom quote by contacting us.
Answer: 50 μl, 100 μl, 200
μl, and 400 μl, at titers of 10^7-10^9 TU/mL.
Answer: You can use
lentivirus to infect or transduce virtually any mammalian cell type. The original HIV genome, from which
lentivirus is derived, has been modified over several generations to make it a safer and more useful gene
delivery vehicle. In the 3rd generation lentiviral vectors that GeneCopoeia uses, the HIV envelope (env)
glycoprotein, which is necessary for infection of CD4+ T-cells, has been replaced with the vesicular stomatitis
virus G (VSV-G) glycoprotein. VSV-G enables lentiviruses to infect virtually all mammalian cell types.
Answer: Lentiviruses
comprise a subtype of retroviruses. Lentiviruses can stably integrate into the host genome in dividing,
non-dividing and post-mitotic mammalian cells, while retroviruses are less active in this scenario. Adenoviruses
can also transduce non-dividing cells, but can’t stably integrate into the host cell’s genome. Adenoviruses also
take much more time to design and prepare. In addition, lentiviruses are much less immunogenic than the
retroviruses and adenoviruses, making lentivirus more suitable for use in most types of cells and animal models.
Answer: One of the key
factors of a successful transduction is the cell type. For example, transduction efficiency is much higher in
actively dividing cells than in non-dividing cells. In addition, transduction of cells works better at lower MOI
(multiplicity of infection) than at higher MOI. MOI is the ratio of the number of lentivirus particles to the
number of cells. For some cell types, the higher the MOI , the larger the volume and higher the titer of
lentivirus is required in order for the experiment to succeed. You can adjust the cell number and add the
appropriate amount of lentivirus according to what has been reported in the scientific literature. If there is
no adequate information in the scientific literature, we recommend performing a preliminary experiment using
gradient dilutions of lentivirus, such as 0.1 μl, 0.3 μl, 0.5 μl, 0.7 μl, 0.9 μl for GeneCopoeia purified particles. Another important consideration for
getting good transduction efficiency is the cell status. Transduction efficiency varies greatly between healthy
cells and unhealthy cells. Therefore, it is essential to keep the cells as healthy as possible. For some cells
with high MOI, you could also include additives such as polybrene to enhance the transduction efficiency.
However, the overall health of the cells itself is always the most essential element.
Answer: Before lentivirus
production starts, you need to first prepare the plasmid DNA using a well-established purification method. Make
sure the plasmid you prepare is of the highest possible quality. You can measure its purity by the absorption
ratio of 260 nm to 280 nm. You should also check the integrity of the plasmid by agarose gel electrophoresis.
GeneCopoeia can provide your clones in transfection-ready format. In rare cases, toxic genes and large fragment
inserts lead to low titers. Next, ensure your lentiviral packaging cell line is well maintained and passaged
regularly, and make sure the culture is free from contamination of bacteria, fungi, and/or mycoplasma. Further,
use an optimized lentivirus packaging system and reagent. We recommend the GeneCopoeia Lenti-Pac™ HIV-Based Lentiviral Packaging system, which is
optimized for production of high viral titer, together with the GeneCopoeia Lenti-Pac™
HIV Expression Packaging Kit. We also recommend using GeneCopoeia 293Ta lentiviral packaging cells (Cat No. LT008). These cells are
guaranteed to provide higher transfection efficiency and lower cell toxicity.
Answer: There are two main GOI-related factors that can affect the lentiviral titer. These are:
Toxicity. Some genes expressed from lentiviruses are toxic to cells and so will sharply reduce the titer.
Insert length. Larger inserts also tend to reduce viral titer.
The issue of insert length must be considered along with the backbone vector size, because the viral titer is
influenced by the total length of the plasmid. Our recommendations for insert size for lentivirus packaging are
shown below. Note that these are only general guidelines:
For Lv200 series vectors (such as Lv201 and Lv206), the insert should be less than 4 Kb.
For most Lv100 series vectors, except for those with eGFP fusions, the insert should be less than 5kb.
For other stripped-down vectors (without reporter or selection gene), the insert plus HIV related parts in the
packing vector should be less than 10 Kb.
Answer: In general, a
lentivirus packaging system contains a gene transfer vector plasmid and a packaging plasmid. The second and
third generation lentivirus packaging systems are both designed to separate the essential genes of the transfer
vector, envelope and packaging components onto different plasmids, thereby reducing the risk of recombination.
When some lentiviral structural proteins must be expressed along with the gene of interest in the second
generation, the vector of the third generation is revamped to make it self-inactivating and tat-independent. The
GeneCopoeia™ HIV-Based Lentiviral Expression System is a modified version of the third generation
self-inactivating (SIN) lentiviral vector system, which incorporates enhanced biosafety features and is
optimized for production of high viral titers.
Answer: The titer of the
GeneCopoeia lentivirus products in each lot are determined by qRT-PCR, which shows the physical number of viral
genomic RNA molecules. The numerical relationship between titer (physical copy number) and transduction unit (TU
or IFU) can be basically summarized using the following formula: TU= Titer (physical copy number)/100. The titer
may vary among different cell types. In general, we recommended conducting a preliminary experiment before your
formal study to ensure the viability of the lentivirus stock and to test the amount needed to transduce the cell
type of interest. For more information regarding titer estimation by transduction, you may download a copy of
the user manual.
Answer: It is safe to use
GeneCopoeia lentivirus. The GeneCopoeia HIV-Based Lentiviral Expression System meets Biosafety Level 2 (BSL-2)
requirements based on the criteria published by the Centers for Disease Control and Prevention (CDC). This
system is a modified version of the third generation of the self-inactivating (SIN) lentiviral vector system,
which incorporates enhanced biosafety features. The lentiviral transfer vector is responsible for transduction
and stable integration into the genome of the host cell, but lacks the elements essential for transcription and
packaging lentiviral particles by itself. Thus, it is self-inactivated, meaning that no unwanted viral
replication and production will happen after the first transfection. Nevertheless, the guidelines for working
with BSL-2 safety category materials must be adhered to. For more information regarding BSL-2, please visit the
CDC website.
Answer: GeneCopoeia offers
a vast number of clones in both HIV-based and FIV-based lentiviral systems, including more than 40,000
human and mouse ORF expression clones, small hairpin RNAi (shRNA)
against genome-wide target genes from human, mouse, rat and other animals, miRNA
inhibitor clones for all known human, mouse and rat miRNAs, promoter reporter clones for
more than 20,000 human and 18,000 mouse promoters, and CRISPR sgRNA
clones for human and mouse. This system provides high expression levels and high efficiency of gene
delivery to virtually all mammalian cell types. The lentiviral expression construct was validated by full-length
sequencing, restriction enzyme digestion, and PCR-size validation using gene-specific and vector-specific
primers. Together with the GeneCopoeia EndoFectin Lenti Reagent (Cat No. EFL1001-01), TiterBoost™
reagent, 293Ta
lentiviral packaging cells (Cat No. CLv-PK-01) and MycoGuard™mycoplasma
detection kit, GeneCopoeia lentiviral products provide high viral titer and are confirmed free of
bacteria, fungi and common mycoplasma contamination.
Answer: Lentivirus has some
level of toxicity to cells. It may cause damage to your cell of interest with either superfluous amounts of
lentivirus, or if the infection were allowed to go on for too long a period of time. In these cases, you can
adjust the multiplicity of infection (MOI) to a lower range. We recommend replacing the old culture medium with
fresh complete medium 4-8 hours post transduction (no later than 12 hours post transduction).
Answer: Lentiviruses can
stably integrate into the host cell’s genome and obtain a consistent level of expression. With a selectable
marker in the lentiviral gene transfer vector plasmid, it is easy to generate a stable cell line using drug
selection. You can use qRT-PCR, western blot or other detection methods to estimate the expression level of your
gene.