Introduction
All-in-One™ qPCR mix with validated primers provide universal qPCR reaction conditions and robust quantitative PCR data. The jointly developed and co-optimized All-in-One qPCR mix and gene-specific primers deliver the entire range of advantages you need without any of the high costs you’ve come to expect:
- Uniform reaction conditions reduce experimental design time enabling earlier journal submissions
- High amplification efficiency and sensitivity even for low-copy genes means reliable quantitation every time
- Absence of non-specific amplification* and no primer-dimers* ensures reproducible and ready-to-publish data
The All-in-One qPCR mix uses high-fidelity hot-start polymerase, an optimized reaction buffer and high-quality dNTPs to enable specific and sensitive amplification from even low-copy RNA (cDNA) or DNA species (Figure 1).
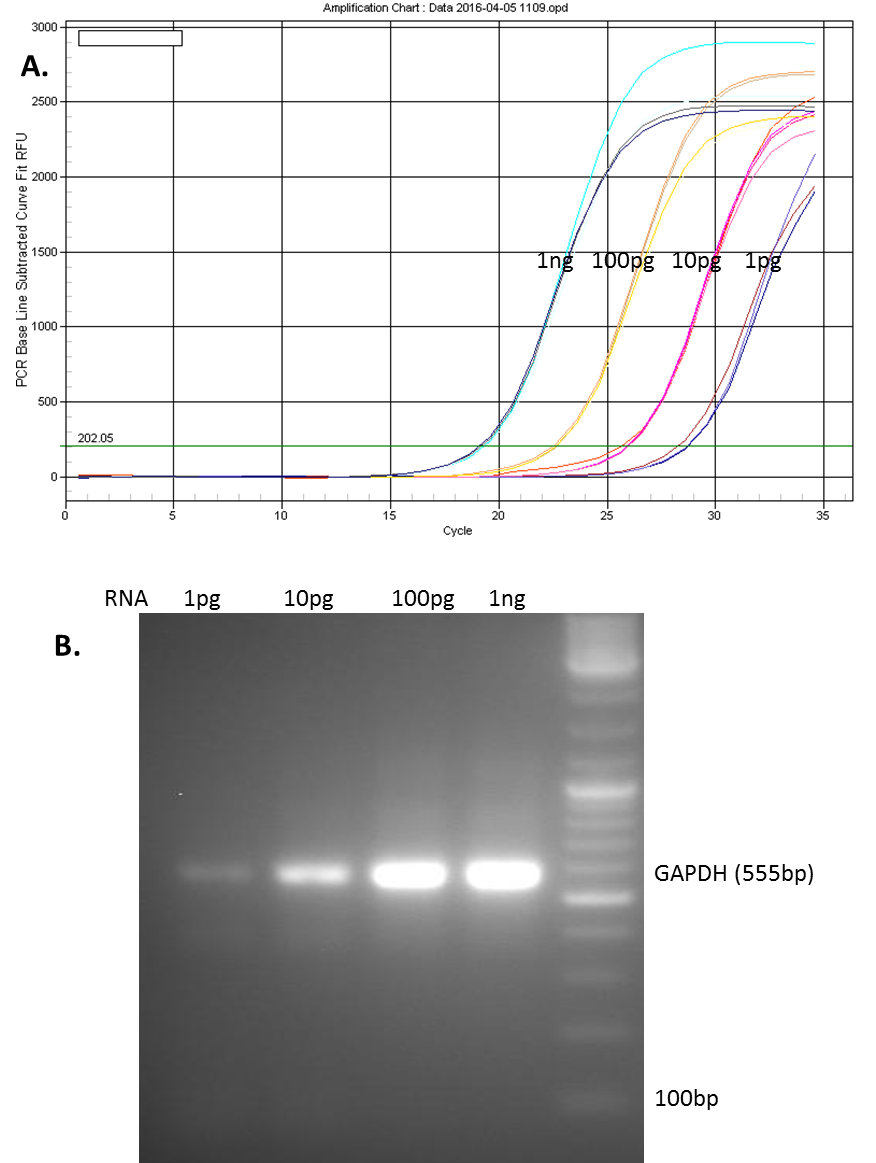
Figure 1. qPCR reaction using the All-in-one qPCR Master Mix. One microgram of total RNA from MCF-7 cells was reverse transcribed using GeneCopoeia All-in-One cDNA synthesize kit. The RT reactions were diluted to 1pg to 1ng of RNA /µl. 1 µl of the diluted RT reactions were used in the subsequent qPCR for 35 cycles using GeneCopoeia All-in-One qPCR Mix (A). The end products were run on 2% Agarose gel (B).
 |
 |
 |
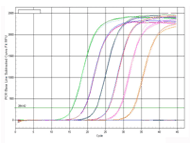
| Amplification curves |
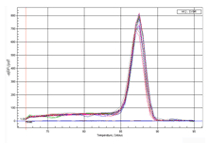
Melting Curves |
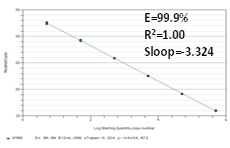
Standard Curve |
Figure 2. The amplification efficiency and detection sensitivity of the 2X All-in-One™ qPCR Mix are assessed by standard curves made by gradient dilution of plasmid DNA from 5×106 to 5 molecules. The peak values from amplification and melting curves show that very high sensitivity can be obtained using All-in-One™ qPCR Mix which can detect as low as 5 molecules. At the same time, high amplification efficiency has also been shown by a good linear relationship among each concentration.
All-in-One qPCR validated primers get the job done 
Eliminate endless adjustments and optimizations with precision qPCR from GeneCopoeia. Just add All-in-One primers and start.
The All-in-One qPCR human- mouse- or rat-specific primers are designed by a proprietary algorithm and validated† for precision performance. Primer validation includes melting curve to ensure amplification of the correct target DNA (figure 2). </p > When used in combination with the All-in-One SYBR® Green qPCR Mix, the All-in-One primers deliver reliable and reproducible high performance in quantitative PCR assays.
 |
 |
| Amplification curves |
 |
| Melting Curves |
Agarose Gel Electrophoresis Validation Result |
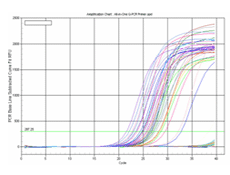
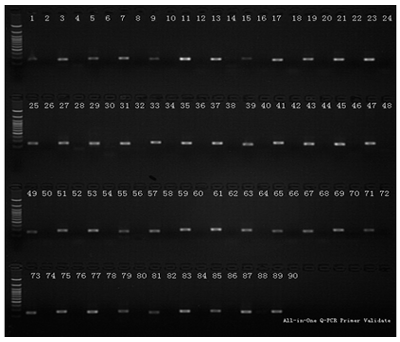
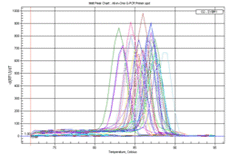
Figure 3. Forty-five pairs of gene-specific All-in-One™ qPCR primers were experimentally validated to yield a single dissociation curve peak and to generate a single amplification of the correct size for the targeted genes. A cDNA pool, containing reverse transcribed products of total RNA from 10 different human tissue (lung, liver, testicle, ovary, spleen, brain, placenta, pancreas, heart and mammary), was used as the qPCR validation template. qPCR was performed using 0.2 µM primer with 2X All-in-One qPCR Mix. Reactions were incubated for 10 min. at 95°C, followed by 40 cycles of 95°C for 10 sec.; 60°C, 20 sec. and 72°C, 15 sec. using Bio-Rad iQ5 Instrument. At the end of the last cycle, the temperature was increased from 72 to 95°C to produce a melting curve. Amplification curves, melting curves and agarose gel electrophoresis (validation result for positive in odd-lane and no template control (NTC) in even lane) shown for the 10 samples.
* Non-specific amplification and absence of primer-dimers are ensured when All-in-One validated PCR primers and PCR mix are used together.
† Primers validated automatically for human, mouse and rat. Inquire for validation of other species.
FAQs
Frequently Asked Questions: qPCR arrays and reagents
Answer: With these arrays, you don’t need to spend time on primer design and validation of genes and miRNAs. These arrays allow you to perform batch qPCR detection of genes or miRNAs simultaneously in the same 96-well or 384-well plate. This greatly simplifies and speeds up your experiments.
Answer: Housekeeping genes are usually used as reference genes, but their expression is not fixed. The expression change of a particular housekeeping gene depends on the experimental conditions. The best method is to choose a series of common housekeeping genes, analyze their expression levels under different conditions, and then select a gene that is stably expressed.
Answer: Your choice of which RT primers to use depends heavily on the experimental design. If you use random primers, reverse transcription will begin at many different sites in the RNA. If you use oligo(dT), reverse transcription will start at the poly-A tail. In quantitative RT-PCR detection of specific genes, we recommend using both random primers and oligo(dT) in one reaction, in order to obtain higher efficiency of reverse transcription. In addition, you can use your own sequence-specific primer to prime reverse transcription in a one-step RT-PCR reaction.
Answer: The DNA polymerase used in these products is a special chemically-modified hot-start enzyme. Incubating for 10 minutes at 95℃ activates the enzyme.
Answer: This kit detects the expression of mature miRNA only.
Answer: The amplification product in miRNA qPCR is about 75bp. The melting temperature of the product is generally between 75℃ and 83℃.
Answer: Most of the GeneCopoeia All-in-One™ miRNA primers are designed to recognize the 3’-end of a template sequence. The matrue miRNA and the pre-miRNA have different sequences at the 3’-end of the base sequence. Therefore, the GeneCopoeia All-in-One™ miRNA primers will not amplify the pre-miRNAs.
Answer: The experimental procedure includes two major steps (Figure 1). In the first step, polyA polymerase is used to add a polyA tail to the 3’ end of the miRNA. Simultaneously, MMLV reverse transcriptase, using a unique oligo(dT) adaptor primer, reverse transcribes the miRNA from the polyA tail. The second step, using the miRNA-specific forward primer and the Universal Adaptor primer as a PCR primer pair, along with a qPCR master mix containing SYBR® Green, specifically detects the reverse transcribed miRNA.
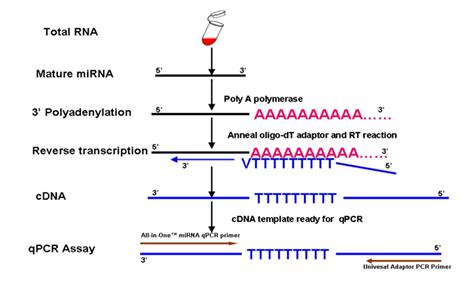
Figure 1. Experimental design used by the GeneCopoeia All-in-One™ miRNA qRT-PCR Detection Kit
Answer: A cDNA pool, containing reverse transcribed products from 10 human tissue total RNA samples, is used as the validation template. qPCR was performed using 0.2 uM primer and the GeneCopoeia All-in-One™ qPCR Mix. The GeneCopoeia All-in-One™ qPCR primers (or the GeneCopoeia All-in-One™ miRNA qPCR primers) are validated to generate a single amplicon of the correct size for the targeted genes and miRNAs and to yield a single dissociation curve peak.
Answer: In combination with the GeneCopoeia All-in-One™ miRNA primers, the GeneCopoeia All-in-One™ miRNA qRT-PCR Detection Kit can detect miRNAs from as little as 10 pg of small RNA or 20 pg of total RNA.
Answer: PolyA polymerase adds poly-A tails to the 3’ end of all miRNAs, which can be reverse transcribed to first strand cDNA in the same tube. The resulting product can be used to detect the expression of any desired miRNAs by qPCR. By contrast, stem-loops are designed to detect just one or a few specific miRNAs, the 3’ end bases of which are paired with their sticky ends. Therefore, to detect such miRNAs, you must select a matching stem-loop. More than one RT reaction may need to be performed at the same time.
Answer: Total RNA has more types of RNA than small RNA, and so provides more templates to amplify in a qRT-PCR detection assay. This can help to improve the amplification sensitivity but could also reduce the amplification specificity.
Answer: The “RT” well contains a spike-in reverse transcription control, which can be used to monitor the efficiency of the reverse transcription reactions. The primer pair pre-loaded in the RT wells specifically amplifies a control cDNA template reverse transcribed from the spike-in exogenous RNA in the sample. The “PCR” well is a positive PCR control, which is used to verify the PCR efficiency by amplifying a pre-loaded DNA template with a specific pre-loaded primer pair. If the RNA sample is of high quality, the cycling program has been correctly run, and the thresholds have been correctly defined, the Ct value of the “RT” well should be 20±3, and the Ct value of the “PCR” well should be 20±2 across all arrays or samples.
Answer: The “GDC” well in each GeneCopoeia ExProfile™ gene qPCR array is a genomic DNA control. This is used to detect the presence of any genomic DNA contamination in each sample during each qPCR reaction. A Ct value for the genomic DNA control of lower than 35 indicates the presence of a detectable amount of genomic DNA contamination. Be sure to remove as much genomic DNA and residual contamination from your RNA samples as possible.









-scaled.jpg)
