*Promotions are valid in the US & Canada only. For international customers, please contact your
. Promotion ends
. Discounts are not valid on previous purchases and cannot be combined with additional discounts.
Product Information AccelerRT® 5G Template Switching RT Enzyme Mix (RNase H-) is a novel RT enzyme that was evolved
in vitro from MMLV RT. The enzyme possesses RNA- and DNA-dependent polymerase activities but lacks RNase H activity. Its efficient template-switching function allows it to be used for full length cDNA products. The engineered enzyme has greatly improved thermal stability, processability and synthesis rates compared to the wild type MMLV RT enzyme.
Applications
- 5′-RACE (Rapid Amplification of cDNA Ends).
- First-strand cDNA synthesis for full length cDNA products.
- Construction of single cell sequencing libraries.
- Discovery and detection of fusion genes.
- Generation of labeled cDNA probes.
- RNA analysis by primer extension.
Performance Data
Template Switching function
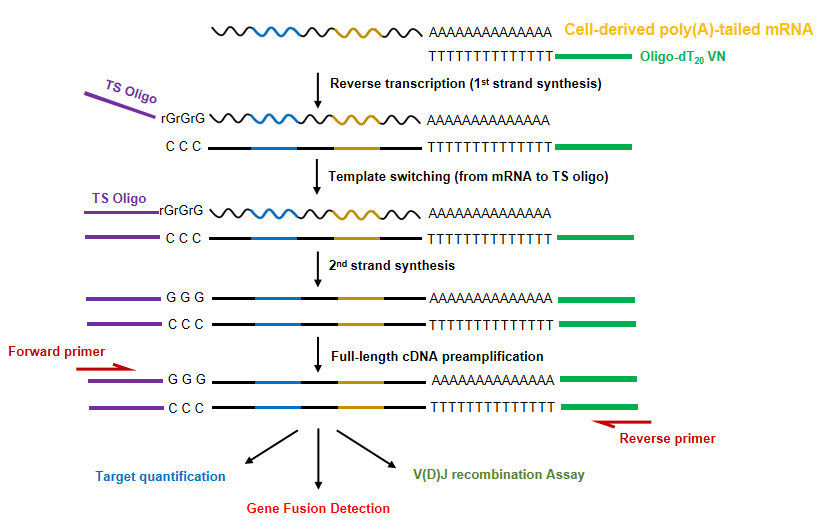
Figure 1. Introduction to the principle of the Template Switching RT Enzyme. The Oligo(dT) VN Primer is used to synthesize the first-strand cDNA. Upon reaching the 5′ end of the RNA template, a few non-template nucleotides are added to the 3′ end of cDNA (Template-switching) using the terminal deoxynucleotidyl transferase (TdT) activity of reverse transcriptase. The second-strand of the cDNA is synthesized using a template-switching oligo. Finally, the full-length cDNA product is obtained by PCR amplification using reverse gene-specific primers and forward Template-switching oligo (TSO)-specific primers.
High Efficiency of Template Switching Synthesis
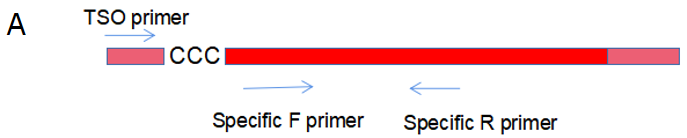
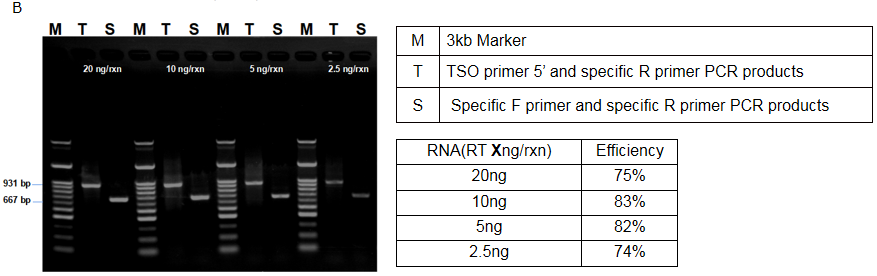
Figure 2. AccelerRT® 5G template Switching reverse transcription reaction was performed using the TSO primer, followed by PCR amplification using the 5 ‘-end portion of the TSO primer and the gene-specific primer(A). The PCR amplification products were subjected to agarose gel electrophoresis(B) and the efficiency of template switch was calculated by product density value and product length (table).
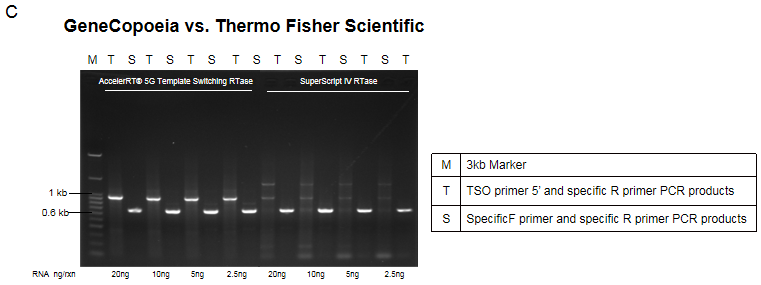 Figure 3. Template Switching Synthesis efficiency of AccelerRT® 5G Template Switching RT Enzyme Mix. 5G Template Switching reverse transcription reaction was performed using the TSO primer, followed by PCR amplification using the 5 ’-end portion of the TSO primer and the gene-specific primer. The PCR amplification products were subjected to agarose gel electrophoresis. SuperScript IV RTase (Thermo Fisher #18090050) was used for comparison.
Figure 3. Template Switching Synthesis efficiency of AccelerRT® 5G Template Switching RT Enzyme Mix. 5G Template Switching reverse transcription reaction was performed using the TSO primer, followed by PCR amplification using the 5 ’-end portion of the TSO primer and the gene-specific primer. The PCR amplification products were subjected to agarose gel electrophoresis. SuperScript IV RTase (Thermo Fisher #18090050) was used for comparison.
Broad thermostability
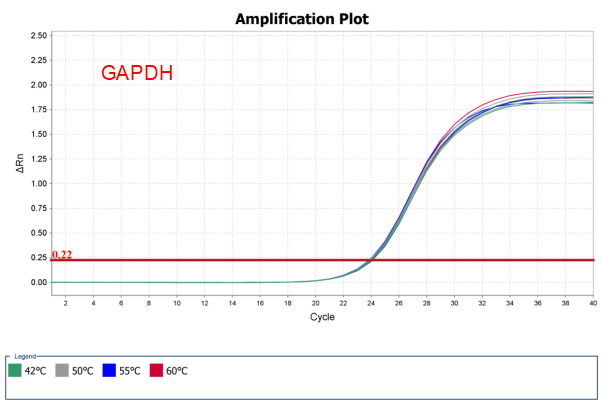
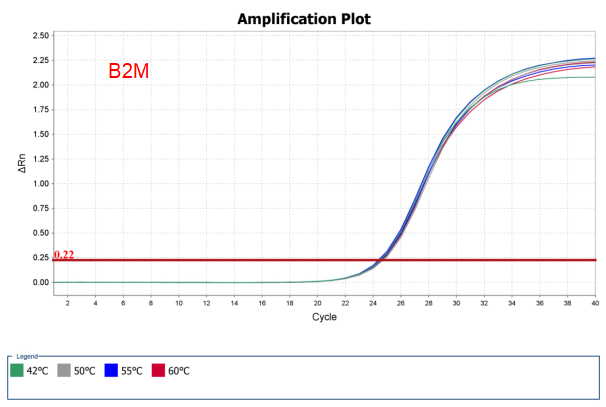
Figure 4. Broad thermal stability of AccelerRT® 5G Template Switching RT Enzyme Mix. 1ng Hela total RNA was reverse transcribed using AccelerRT® 5G Template Switching Reverse Transcriptase at different temperatures from 42 ℃ to 60℃. The GAPDH and B2M cDNAs were amplified with BlazeTaq™ SYBR® Green qPCR mix 2.0(Cat.# QP031).
Exceptional RNA template sensitivity
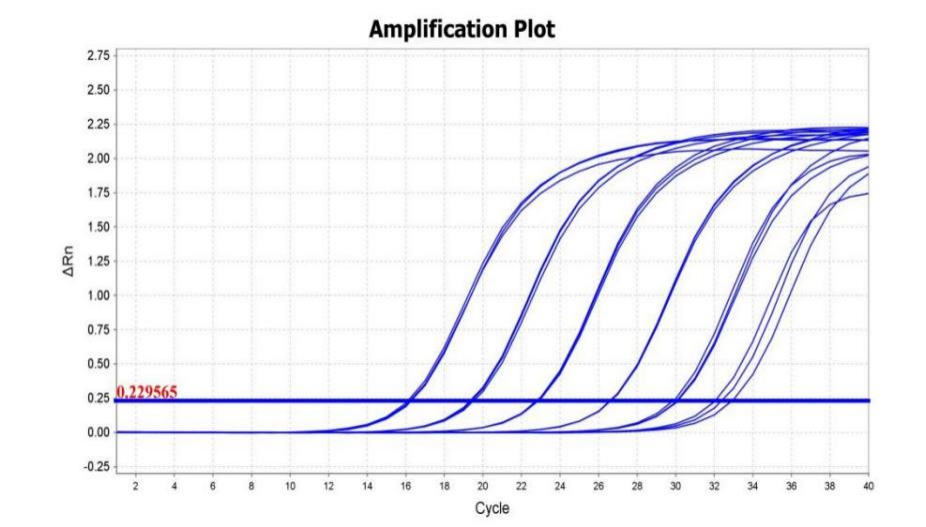
Figure 5. First-strand cDNA was generated from 100 ng to 1 pg of total RNA from Hela cells using AccelerRT® 5G Template Switching RT Enzyme Mix. The synthesized cDNA was used as a template for qPCR using BlazeTaq™ SYBR® Green qPCR mix 2.0(Cat.# QP031).
High efficiency of reverse transcription
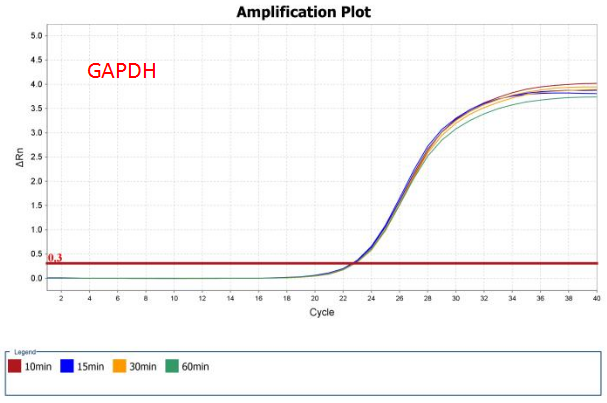
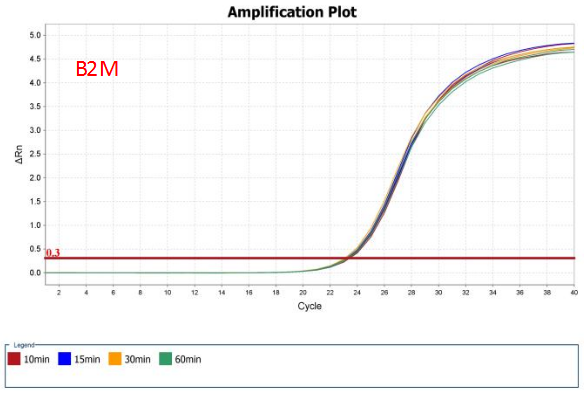
Figure 6. RT efficiency of AccelerRT® 5G Template Switching RT Enzyme Mix was demonstrated using 1ng Hela total RNA reverse transcribed for 10min, 15min, 30min and 60min. The GAPDH and B2M cDNAs were amplified using BlazeTaq™ SYBR® Green qPCR mix 2.0(Cat.# QP031).
Consistent performance in the presence of a variety of inhibitors
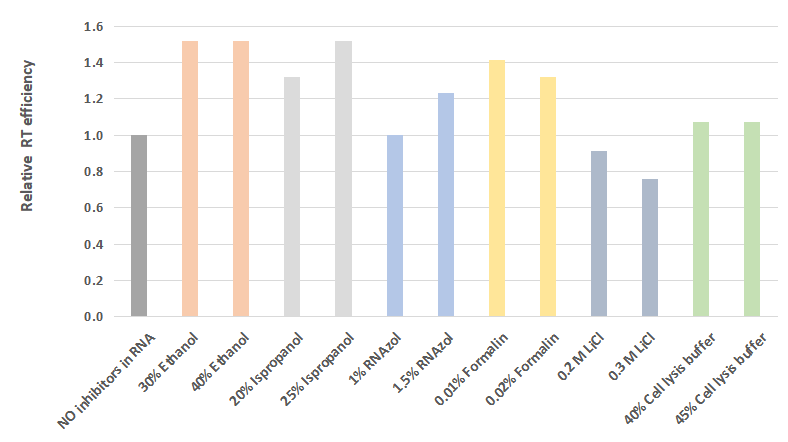
Figure 7. Various inhibitors were added to total HeLa RNA, then was used in a reverse transcription reaction with AccelerRT® 5G Template Switching RT Enzyme Mix, The synthesized cDNA was used as a template in subsequent qPCR using BlazeTaq™ SYBR® Green qPCR mix 2.0(Cat.# QP031).
Effective amplification of cDNA Synthesis
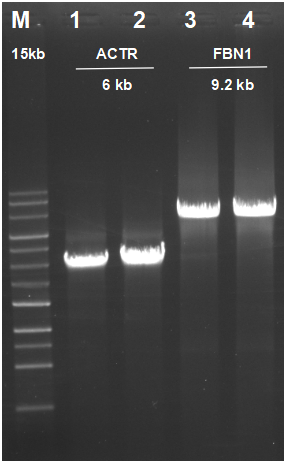
Figure 8. Total RNA from Hela cells was used in a reverse transcription reaction with AccelerRT® 5G Template Switching RT Enzyme Mix. The synthesized cDNA was used as a template in subsequent PCR using the UltraHiPF®
DNA Polymerase Kit (Cat.# PC018).
Discovery and detection of fusion genes
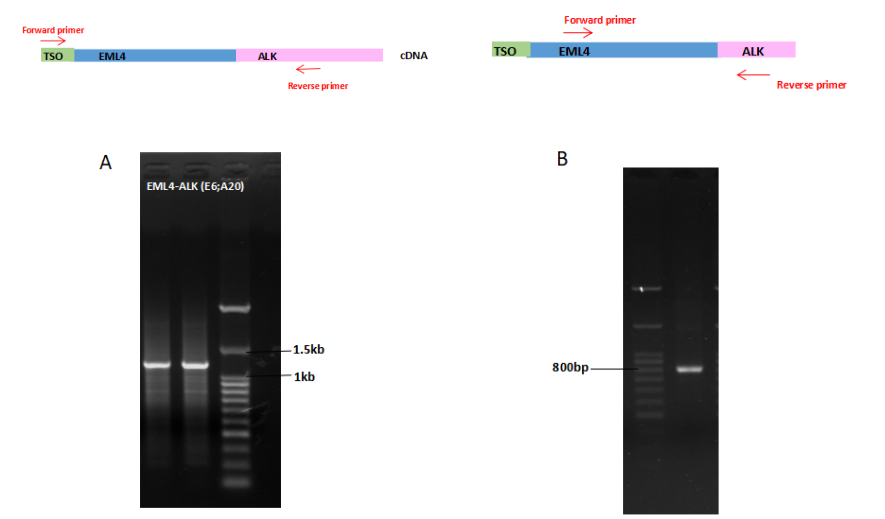
Figure 9
. Total RNA from H2228 cells was used in a reverse transcription reaction with AccelerRT® 5G Template Switching RT Enzyme Mix. The synthesized cDNA was used as a template in subsequent PCR using the 5 ‘-end portion of the TSO primer and the gene-specific primer ALK gene(A). The amplification products in Figure A was verified by amplification with the EML4 forward primer and the ALK reverse primer(B).
* IP protected and AccelerRT® 5G Trademark owned by GeneCopoeia, Inc.