Product Information
GeneCopoeia’s CytoCt™ RT-qPCR System enables you to analyze gene expression by real-time RT-qPCR directly from 10-100,000 cultured cells either in tubes or 96-well plates without any RNA purification, providing enhanced convenience, speed, throughput, data reliability, and sensitivity. RNA is traditionally extracted and purified from cultured cells before gene expression quantification and profiling analysis by RT-qPCR assays. The CytoCt™ RT-qPCR System skips the RNA extraction step and prepares cell lysates directly from cells cultured in 96-well plates or other formats. With GeneCopoeia’s CytoCt™ RT-qPCR System, gene expression analysis can be completed in about 90 minutes using a cell lysis buffer followed by either a two-step or a one-step RT-qPCR workflow. The system includes CytoCt™ Cell Lysis kits, CytoCt™ cDNA Synthesis kits, and CytoCt™ One-Step RT-qPCR kits.
Advantages
- Convenient RT-qPCR directly from cultured cells without any RNA purification
- Sensitive detection of gene expression from 10-100,000 cultured cells
- High-throughput and enhanced speed with direct cell lysis in 96-well plates or in tubes
- Robust performance with system optimization from cell lysis to RT-qPCR reactions
Simple Workflow
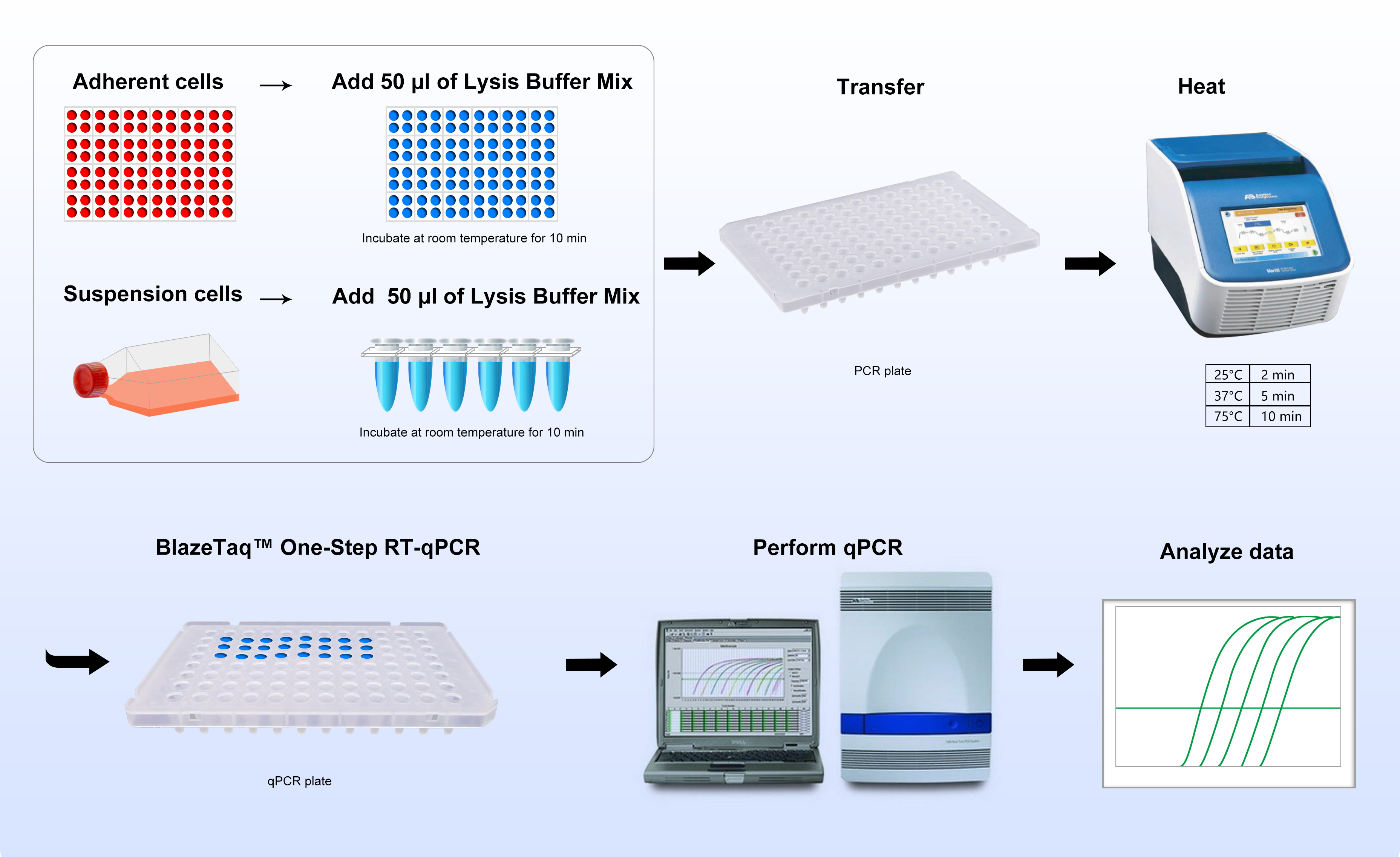
Simply grow cells to desired density, then add lysis solution and incubate at room temperature for 10 minutes. Next, transfer to a PCR plate, followed by heating for 2 minutes at 25°C, 5 minutes at 37°C and then for 10 minutes at 75°C. The lysates are ready for RT-qPCR reactions or can be stored for future use.
Performance Data
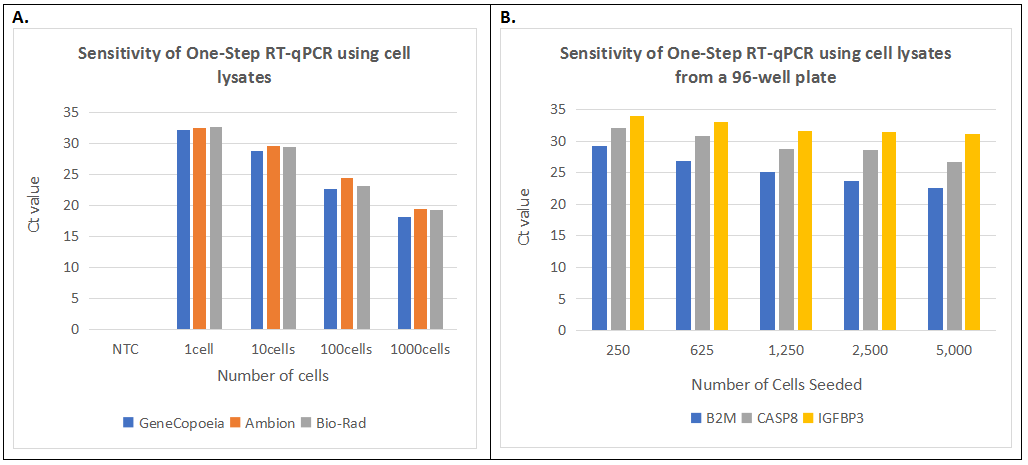
Figure 1. Sensitivity of One-Step RT-qPCR using cell lysates from tubes or 96-well plates. A. One-Step RT-qPCR analysis of the GAPDH gene using cell lysates from HCT116 cells lysed with GeneCopoeia’s, Ambion’s, or Bio-Rad’s lysis buffers. 5 µl of the suspended cells at different concentrations of 5 to 5,000 cells/µl were mixed with the lysis buffers and processed following the user manual of each manufacturer. 2µl of cell lysate (estimated to be equivalent to 1-1,000 cells/reaction) was used in each One-Step RT-qPCR reaction. B. H1299 cells were seeded in 96 well plates with a gradient of 250 to 5,000 cells per well. After culturing for 96 hours, the final cell numbers per well were estimated to be around 2,500 to 50,000 cells depending on the initial seeding densities. Cells in each well were mixed with 50µl lysis buffer plus DNase I and processed following the user manual. 2µl of cell lysate (estimated to be equivalent to 10-200 cells/reaction) were used for the One-Step RT-qPCR reactions.
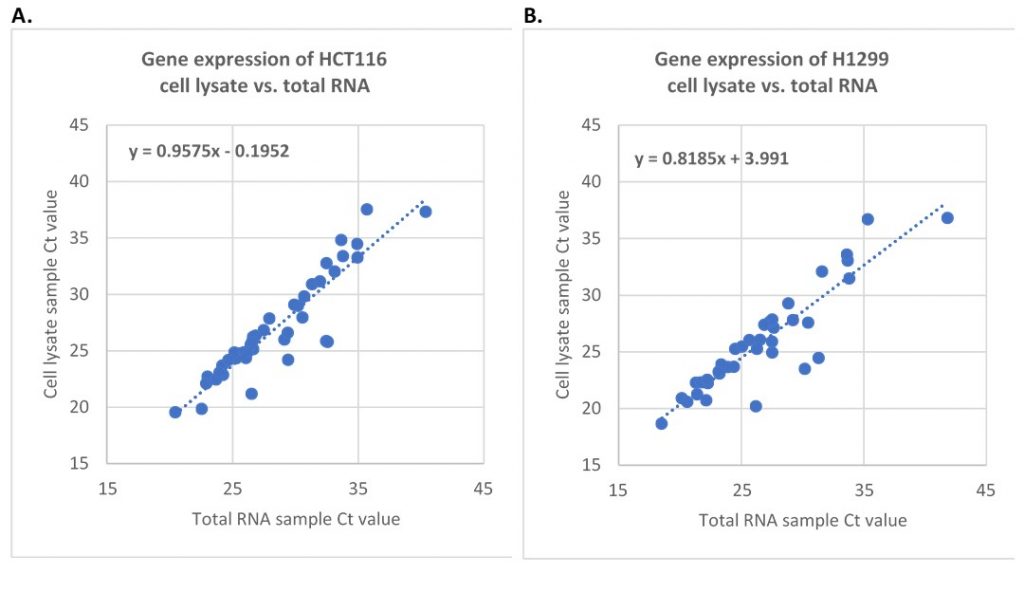
Figure 2. One-Step RT-qPCR of gene expression using either the total RNA or CytoCt™ buffer lysed-cell lysate from the same number of cells. There were about 1,500 cell equivalents per reaction. A. 42 genes from HCT116 cells. B. 37 genes from H1299 cells.
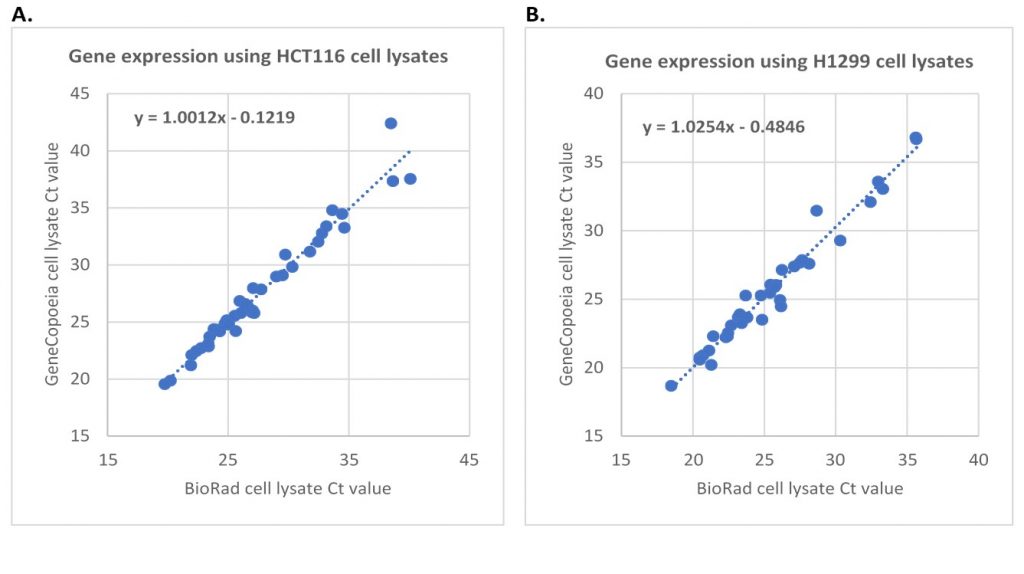
Figure 3. One-Step RT-qPCR of gene expression using cell lysates from the same number of cells lysed with either GeneCopoeia or BioRad lysis buffers. There were about 1,500 cell equivalents per reaction. A. 43 genes from HCT116 cell lysate. B. 37 genes from H1299 cell lysates.
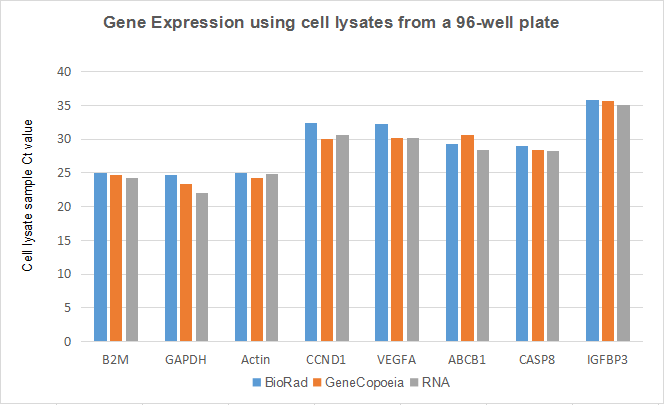
Figure 4. RT-qPCR detection using cell lysates in a 96-well plate. 3,000 – 5,000 of HEK293 cells were seeded per well in a 96-well plate and cultured until reaching 70% to 90% confluency. Cells were either lysed with GeneCopoeia’s and Bio-Rad’s lysis buffers, or RNA was extracted. Small amounts of cell lysates (estimated to be equivalent to 200 cells/reaction) were used for the One-Step RT-qPCR reactions to detect the expressions of eight genes.