Product Information
Tumor-associated autoantibodies (TAAbs) are promising biomarkers for early cancer detection, monitoring, and prognosis because the immune system creates them in response to tumor-associated antigens (TAAs) long before symptoms appear. While individual autoantibodies often lack sufficient sensitivity, panels or combinations of several TAAbs (e.g., against p53, NY-ESO-1, CAGE, HSP70) significantly improve diagnostic accuracy for various cancers like lung, breast, and prostate cancer, offering a non-invasive “liquid biopsy” for early diagnosis.
Role in Cancer Diagnosis & Prognosis
Early Detection: TAAbs can appear in the blood before a tumor is clinically evident, acting as an early warning signal for cancer, particularly breast cancer.
Biomarkers: The detection of TAAbs in serum can reflect the tumor’s presence and molecular state, potentially predicting recurrence or response to treatment.
Advantages: Autoantibodies are stable in blood, and their detection offers a less invasive method than tissue biopsies, potentially overcoming mammography limitations.
Specificity/Sensitivity: Detecting single autoantibodies often lacks accuracy; however, combining multiple autoantibodies (creating an “autoantibody signature”) dramatically increases sensitivity and specificity.
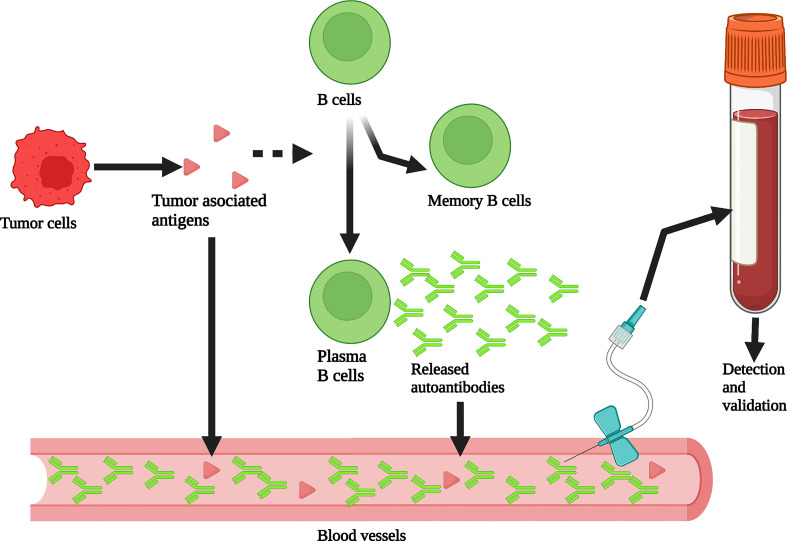
Fig. 1 Production and detection of peripheral blood autoantibodies. Tumor associated antigens (TAAs) produced by tumor cells stimulate B cells to differentiate into memory B cells and plasma B cells that can produce autoantibodies. Autoantibodies can be detected from plasma or serum to assist in breast cancer screening. (Ref. Yang et al.)
Reference:
The 60 TAAs included in the OmicsArray™
Tumor associated antigen array panel were chosen based on the thorough review of published and ongoing studies. The superior-quality purified antigens were coated onto the 3D surface of microchips to ensure that antigens retain their natural conformations. Internal Ig and anti-Ig controls are included in each array for normalization and quantification purposes. Additionally, the utilization of fluorescent 2nd antibodies in our microarrays allows for the detection of two types (IgG, IgM, or IgA) of antibodies on the same array, minimizing the requirement of sample needed.
In addition to premade arrays, arrays containing customized sets of antigens are available, as well as array processing kits, array profiling services and data analysis. To order premade or custom arrays, please contact us.
GeneCopoeia’s OmicsArray™ Tumor associated antigen array is part of the GeneCopoeia OmicsArray™ Antigen Microarray family.
To Order
Premade Antigen Arrays
The listed price applies to academic customers. For custom arrays and services pricing, please contact us.
Antigen microarray processing kits and accessories
Custom services
GeneCopoeia offers custom antigen microarray services in the following areas:
- Custom array printing. GeneCopoeia will create custom antigen microarrays built to your specifications.
- Sample processing. Send us your blood, plasma, tissue, or other biological sample and we will prepare it for processing and incubation with any of our premade antigen microarrays or custom-built antigen microarrays for autoantibody profiling and other applications. For information on sample types to submit, consult the FAQ
- Data analysis. Once samples are processed and incubated with an antigen microarray, we will analyze the raw data. The standard analysis service includes: 1) An Excel file of the Net Signal Intensity (NSI) for each antigen on the array, normalized to internal controls; and 2) a heat map
Additional analysis services, including proteomic analysis, pathway analysis, and more, are also available.
To inquire about custom antigen microarray products and services, please fill out our custom quote request form.
Technology overview
Advantages of OmicsArray™ Antigen Microarrays
- Largest collection of pre-made whole-protein antigen microarrays on the market.
- Largest number of whole-protein antigens specifically focused on autoimmune disease research.
- Best combination of number of antigens per array (up to 120) with number of samples that can be processed per slide (up to 15).
Technology overview
GeneCopoeia’s OmicsArray™ antigen microarrays contain up to 120 purified proteins spotted onto nitrocellulose filters, which are adhered to glass slides. In addition, 8 spots are included for normalization. Each slide carries 16 identical arrays, and so can be used to process up to 15 samples simultaneously as well as a negative control. As little as 1 μL serum or 50 μL of other biofluids are needed for each sample.
As shown in Figure 1, arrays are incubated with patient samples, and any antibodies in the samples bind to their cognate antigens on the array. The arrays are washed to remove unbound antibodies and other proteins, then co-incubated with Cy3- and Cy5-labeled secondary antibodies. The dual labeling strategy is intended to distinguish between immunoglobulin (Ig) subtypes present within samples. For example, a Cy3-labeled anti-IgG secondary antibody is used to detect IgG antibodies, and a Cy5-labeled anti-IgM secondary antibody is used to detect IgM antibodies. Fluorophore-labeled secondary antibodies are available for detecting IgA, IgD, IgE, IgG and IgM immunoglobulins, as well as IgG subclasses IgG1, IgG2, IgG3, and IgG4.
After washing to remove unbound secondary antibodies, signals are detected using a microarray scanner (e.g., GenePix® 4000B, InnoScan 710, or equivalents). The raw data is then analyzed using GenePix® Pro 7.0, or Mapix software.
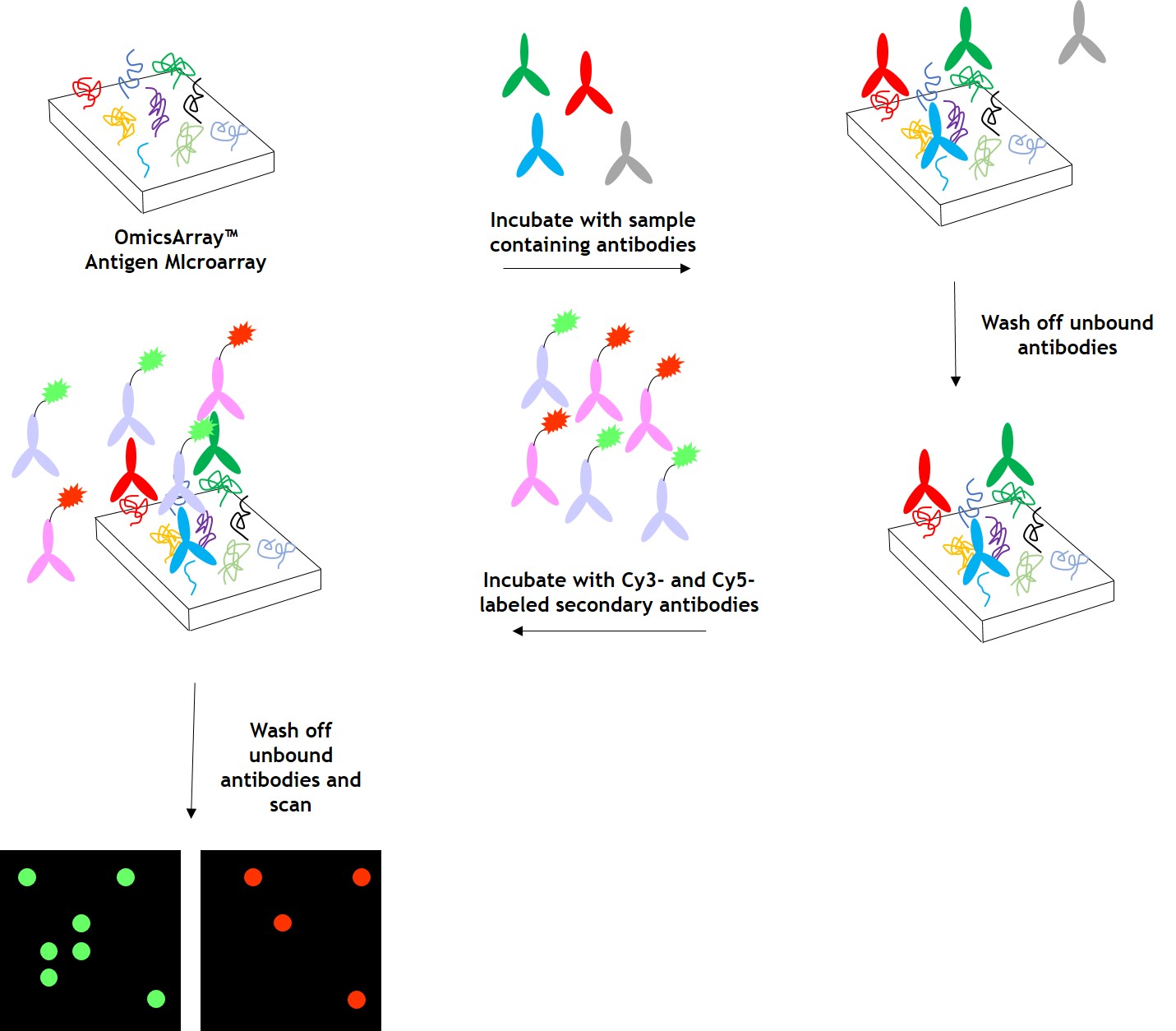
Figure 1. Workflow for detection of antibodies in samples using GeneCopoeia’s OmicsArray™ antigen microarrays.
Data analysis, FAQs


