| Ed Davis, Ph.D. |
Introduction
Lentiviral vectors have proven invaluable for introducing genetic material into mammalian cells, either in culture or whole animals (reviewed in Mátrai, et al., 2010). Lentiviruses, primarily derived from human or feline immunodeficiency viruses (HIV or FIV, respectively), infect most cell types, including those that are difficult-to-impossible to transfect with plasmids. GeneCopoeia offers every one of our human and mouse ORF, promoter, shRNA, precursor microRNAs, microRNA inhibitor, and CRISPR sgRNA clones in lentiviral vectors. In addition, we offer our Lentifect™ pre-made and custom lentiviral particles for our customers, which will save you time and effort over having to package the viral particles yourself. Some of our customers, however, have many questions on how to best use Lentifect™ particles in transduction experiments, particularly how large a titer of particles to purchase. The answer to this question is dependent on what is the best multiplicity of infection (MOI) to use for a given cell line. In this Technical Note, we offer recommendations for which MOI to use for many common cell lines, which will help guide you toward the correct volume of Lentifect™ lentiviral particles to order.
Multiplicity of infection
In order to know what volume of GeneCopoeia Lentifect™ lentiviral particles you need to use for a particular cell line, you need to know the correct MOI for that cell line. MOI is a very simple concept: It is the ratio of the number of viral particles used to infect cells to the actual number of cells. The titer of GeneCopoeia Lentifect™ lentiviral particles is given as transduction units (TU) per milliliter. 1TU = 1 infectious particle, so if 106 TU are used to infect 106 cells, then the MOI = 1. If 5 x 106 TU are used to infect the same number of cells, then the MOI = 5.
What MOI do I use for my cell line?
The ability of lentiviruses to infect cells varies greatly among different cell lines, and so affects the MOI. GeneCopoeia has determined the optimal MOI to use for many cell lines. A list of MOIs to use for these cell lines is displayed in Table 1.
Of course, in order to determine your MOI, you need to first know your viral titer, or the concentration of infectious particles. GeneCopoeia indicates the viral titer as TU. Typically—if the insert in the lentiviral vector is less than 4-5 kb and is not toxic to cells—we provide purified lentiviral stocks in the range of 107-109 TU per ml. Therefore, let’s say you are working with the breast adenocarcinoma cell line MCF-7.
According to Table 1, the optimal MOI to use is 2. So, if you purchase 50 microliters of viral particles and the titer is 108 TU/ml, then you should have a total of 0.05 ml x 108 particles ml-1 = 5 x 106 particles. To achieve an MOI of 2, then, 50 microliters at 108 TU/ml will provide enough particles to transduce 5 wells of a 24-well dish, each well containing 4 x 105 MCF-7 cells. If your cell line requires a much higher MOI,then you might want to consider purchasing more particles-standard amounts come in 100 microliter or 200 microliter volumes. Naturally, higher MOIs will require higher volumes of viral particles.
| Cell line | Tissue | Cancer/cell type | Species | MOI |
| A431 | Epithelial | Carcinoma | Human | 5 |
| A549 | Lung | Carcinoma | Human | 5 |
| Astrocytes | Nervous system | Primary | Human | 1 |
| B16-F10 | Epithelial | Melanoma, metastatic | Mouse | 5 |
| BMM | Bone Marrow | Primary | Human | 8 |
| BxPC-3 | Pancreas, epithelial | Adenocarcinoma | Human | 10 |
| H3255 | Lung | Carcinoma, NSCLC | Human | 10 |
| HCT116 | Colon | Carcinoma | Human | 5 |
| HeLa | Cervix | Carcinoma, epitheloid | Human | 3 |
| HEK293T | Kidney | Tumor | Human | 5 |
| Hepa1-6 | Liver | Carcinoma | Mouse | 3 |
| HMVEC | Endothelial | Endothelial, microvascular | Human | 100 |
| HT-29 | Colon | Adenocarcinoma | Human | 3 |
| HUVEC | Umbilicus | Endothelial cells | Human | 100 |
| Jurkat | Blood | Leukemia, Acute T Cell | Human | 10 |
| LLC-1 | Lung | Carcinoma | Mouse | 6 |
| LNCaP | Prostate | Carcinoma | Human | 5 |
| MM200 | Skin | Melanoma | Human | 5 |
| MCF-7 | Breast | Adenocarcinoma | Human | 2 |
| MDA-MB-231 | Breast | Adenocarcinoma | Human | 1 |
| MM-AN | Skin | Melanoma, metastatic | Human | 16 |
| MMC | Breast | Carcinoma | Mouse | 4 |
| MRC-5 | Lung, embryonic | Fibroblasts | Human | 1 |
| NB4 | Blood | Leukemia, acute promyelocytic | Human | 10 |
| PC12 | Adrenal gland | Pheochromocytoma | Rat | 20 |
| SKOV-3 | Ovary | Adenocarcinoma | Human | 15 |
| U-2 OS | Bone | Osteosarcoma | Human | 5 |
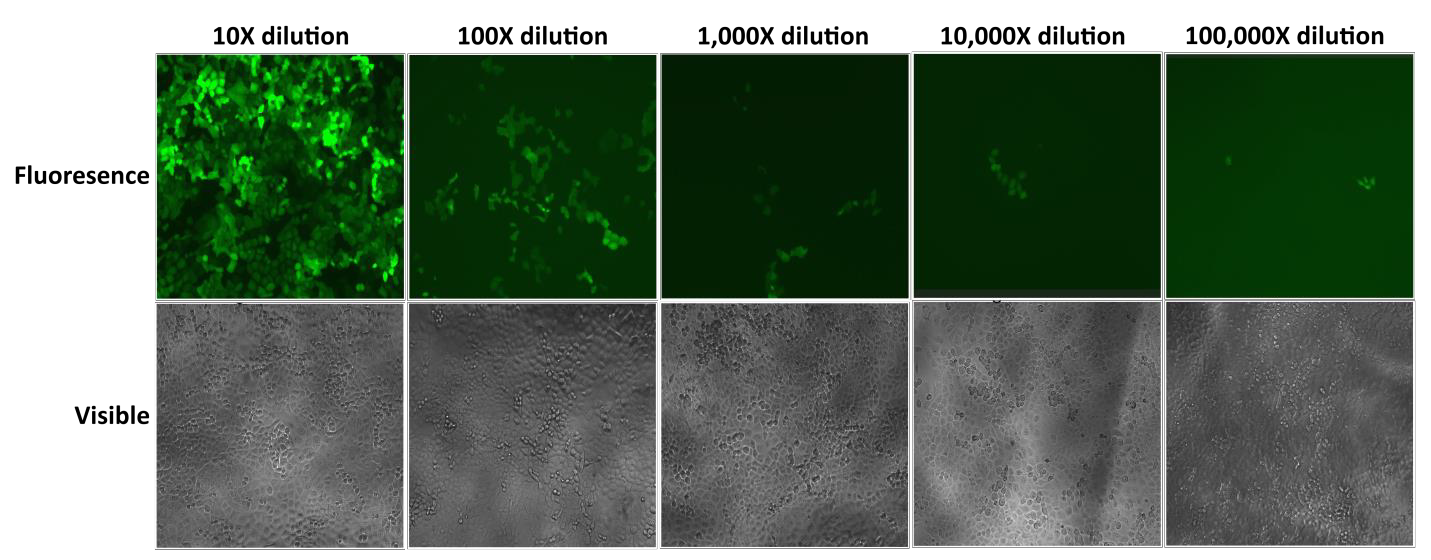
Note that the MOI values listed in Table 1 should only be used as a guide for how large a volume of lentiviral particles to purchase. Once you receive your particles from GeneCopoeia, we recommend testing the transduction efficiency of your cell lines using MOI values of 0.3, 1, 3, 5, and 10 on a small number of cells. In addition, if your cell line is not listed in Table 1, then it will be essential to determine your optimal MOI manually. We recommend performing serial dilutions of your lentiviral particles and using those dilutions to infect your cells, as is shown in Figure 1.
Figure 1 Test of serial dilutions of Lentifect™ lentiviral particles. 1 day before transduction, 1 x 104 H1299 cells were seeded into wells of a 96-well plate. On day 2, viral particles expressing GFP were serially 10-fold diluted in 100 microliters of culture medium as indicated. The medium was aspirated, and diluted particles were added to the cells. eGFP fluorescence was photographed with a fluorescence microscope 72 hours post-transduction.
Finally, if your cell line requires a high MOI, you might be able to reduce it using one of several treatments. One is polybrene (hexadimethrine bromide), a cationic polymer that reduces charge repulsion between viral particles and the cell membrane (Davis, et al., 2004). Another of these methods is to use magnetic beads that capture the viral particles (Hughes, et al., 2001). For example, use of magnetic beads can reduce the required MOI of MM-AN cells from 16 to 4. We have also found that using drug selection with a plasmid-borne marker such as puromycin, allows you to establish a stable cell line with the lentiviral vector randomly integrated into the genome.
Contact us today to find out more about GeneCopoeia Lentifect™ lentiviral particles or to get a quote:1-301-762-0888. You may also visit us at https://www.genecopoeia.com.
References
Davis, et al. (2004). Charged Polymers Modulate Retrovirus Transduction via Membrane Charge Neutralization and Virus Aggregation. Biophysical Journal 86, 1234.
Hughes, et al. (2001). Streptavidin paramagnetic particles provide a choice of three affinity-based capture and magnetic concentration strategies for retroviral vectors. Molecular Therapy 4, 623.
Mátrai, et al. (2010). Recent Advances in Lentiviral Vector Development and Applications. Molecular Therapy 18, 477.
| Copyright ©2014 GeneCopoeia, Inc. www.genecopoeia.com TNLV1-111014 |