| Ed Davis, Ph.D. |
Introduction
Lentivirus and Adeno-associated virus (AAV) have proven invaluable for introducing genetic material into mammalian cells, either in culture or whole animals. Both systems are highly amenable for many basic research applications, such as protein overexpression, antibody production, and gene knockout, and both hold promise for gene therapy. However, each viral system has its own unique advantages and disadvantages, depending on the application. GeneCopoeia offers extensive product lines for both lentivirus and AAV, providing you with powerful and flexible options for delivering DNA into cells. In this Technical Note, we describe the technologies behind GeneCopoeia’s Lentifect™ lentivirus and AAVPrime™ AAV product lines, and discuss the merits of each technology for various applications in order to help you choose which system best suits your needs.
Why use viruses for DNA delivery?
One of the most common ways to deliver DNA to cells is through plasmid-based transfection, in which cells are treated with chemical compounds like calcium phosphate, or with lipid-based reagents. However, plasmid transfection is not always desirable or practical. For example, some cells are very difficult or impossible to transfect, whereas most cultured cells support infection (also known as transduction) by either lentivirus or AAV. Secondly, plasmid transfection cannot be used for in vivo DNA delivery, but viral transduction can. Both lentivirus and AAV can be used for a multitude of applications, such as protein expression from open reading frames (ORFs), gene knockdown by RNA interference (RNAi) mediated by short hairpin RNA (shRNA), luciferase and other reporter gene assays, and gene knockout mediated by the clustered, regularly-interspaced, short palindromic repeats-Cas9 (CRISPR-Cas9) system. Many of these applications for viral vectors have been successfully carried out in cultured immortalized mammalian cell lines, primary cell culture, animal models, and in gene therapy on human patients.
Lentiviral systems
The term “Lentivirus” refers to the class of retroviruses that includes human immunodeficiency virus (HIV), and, in fact, most lentiviral systems currently in use are derived from HIV (reviewed in Mátrai, et al., 2010; Sakuma, et al., 2012). Lentiviruses carrying transgenes integrate into the genome upon infection, and so permit stable expression in both dividing and non-dividing cells.
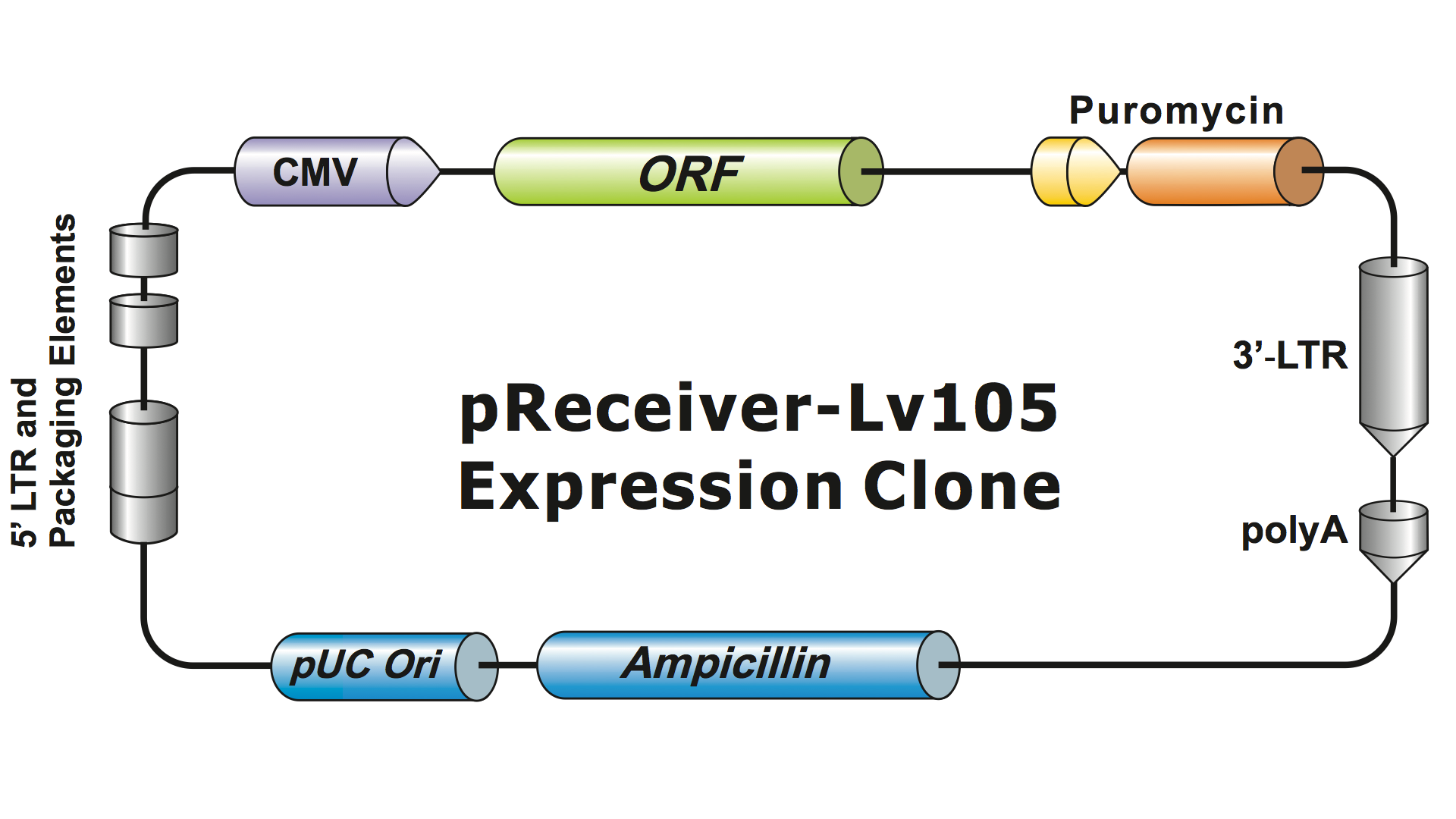
Lentiviral systems have been highly modified from HIV over several generations to make them safe to handle and useful for applications. GeneCopoeia uses the 3rd generation lentiviral system (Dull, et al. 1998), which requires four separate plasmids to produce infectious viral particles (virions). A GeneCopoeia lentiviral plasmid carries only the gene of interest (GOI) to be expressed, an antibiotic selection gene, and the packaging signal sequences. Sometimes the clone will also include a fluorescent reporter gene. The GOI-containing plasmid, with the selectable marker and reporter gene, can accommodate inserts up to about 5-6 kb. (Figure 1), although viral titers drop dramatically when insert sizes exceed about 4 kb.
Figure 1. Example of a GeneCopoeia lentiviral expression clone
The additional plasmids express the retroviral elements needed for packaging and integration (gag, pol, env, Rev). When all four plasmids are expressed in packaging cells, the GOI RNA is incorporated into particles. Because the retroviral proteins are expressed only during packaging, infected cells cannot produce infectious virus. In addition, GeneCopoeia’s Lentifect™ lentiviral particles contain a deletion in the U3 region of the LTRs, making them self-inactivating (SIN). The SIN deletion prevents lentiviral replication in the rare event that all four plasmids recombine to create a single active virus plasmid. Therefore, lentiviral particles are safe to handle, with virtually no chance of generating pathogenic virus.
In addition to the enhanced safety modifications, GeneCopoeia’s Lentifect™ lentiviral particles carry the vesicular stomatitis virus G (VSV-G) glycoprotein in place of the wild type HIV env gene, which changes the tissue tropism of the virus from CD4+ T-cells to virtually any mammalian cell type. As such, lentiviruses are a widely-used system for mammalian cell culture, animal models, and gene therapy applications.
Because lentiviral vectors have been modified in these ways, GeneCopoeia’s Lentifect™ lentiviral systems have enhanced safety, and have the following features that make them highly useful for DNA delivery:
- They infect nearly all mammalian cell types
- They can be used to deliver relatively large DNA sequences-up to about 5-6 kb in length
- They can be used to generate stable cell lines, or drive stable gene expression in organs and tissues in vivo, due to integration of the transgene at random locations in the genome
GeneCopoeia’s Lentifect™ lentiviral particles provide high titers, enabling researchers to efficiently express genes of interest (Figure 2).
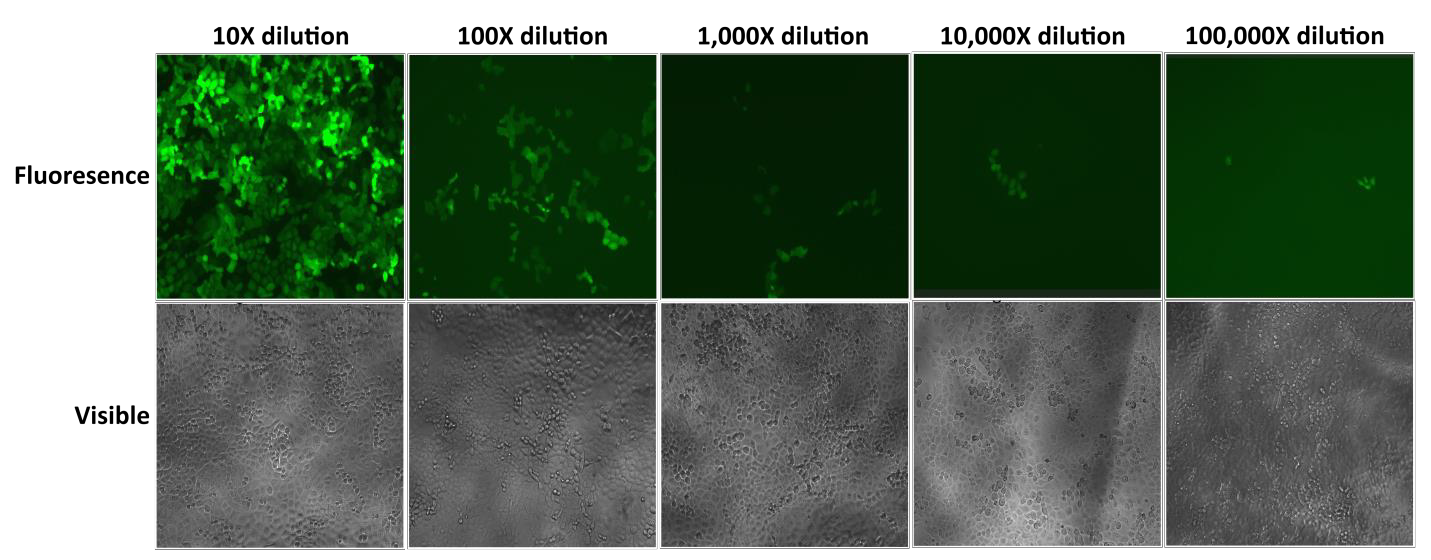
| Figure 2. Test of serial dilutions of Lentifect™ lentiviral particles. eGFP fluorescence was photographed with a fluorescence microscope 72 hours post-transduction. |
| Product/service | Description |
| Lentiviral clones and cloning vectors | Pre-made and custom clones carrying ORFs, promoters, shRNAs, miRNA 3’ UTRs, precursors, and inhibitors, sgRNAs, and more. Available with multiple promoters, tags and reporters. Vectors for do-it-yourself cloning of sequences of interest. |
| Lentifect™ lentiviral particles | Pre-made and custom-packaged, ready to use lentiviral particles. Produced from GeneCopoeia’s extensive, genome-wide clone collections or from customer-submitted clones. |
| Lenti-Pac™ Lentiviral Packaging Reagents | Complete system of reagents for do-it-yourself lentiviral particle production. Includes packaging plasmids, packaging cell line, particle concentration solution, and titration kit. |
AAV-based systems
In contrast to the RNA genome of lentiviruses, AAV has a single stranded DNA genome (Samulski and Muzyczka, 2014). Also, AAV is not derived from a pathogen; rather it is a contaminant of adenovirus, with no pathogenic conditions ascribed to it. Naturally-occurring AAV integrates into the genome like lentivirus, but only at the AAVS1 locus on chromosome 19. This site is also known as “Safe Harbor”, because it is commonly used for transgene insertion without harming cells (DeKelver, et al., 2010).
Like lentivirus, AAV has been modified for safety and usability. AAV requires co-infection with a helper virus. The AAV genome is 4.7 kb long and contains 2 genes, Rep and Cap, which are required for viral replication and integration. In AAVPrime™, Rep and Cap are deleted, leaving only the 145 bp 5’ and 3’ inverted terminal repeats (ITRs). Deleting Rep and Cap serves three purposes: 1) It eliminates the ability of a helper virus to permit AAV-infected cells to produce new AAV virions; 2) It permits insertion of genes up to approximately 4 kb long (although viral titer diminishes with transgenes longer than 3 kb); and 3) It virtually eliminates the ability of AAV to integrate. The small size of AAV presents challenges for some applications. For example, for CRISPR genome editing, researchers use Cas9 nuclease from S. aureus, because it is about 30% shorter than the more commonly used S. pyogenes Cas9 (Ran, et al., 2015).
For packaging, Rep and Cap have been moved to a different plasmid, and the Adenovirus helper genes are placed on a third plasmid, making the entire packaging system safe and helper virus-free (Figure 3).
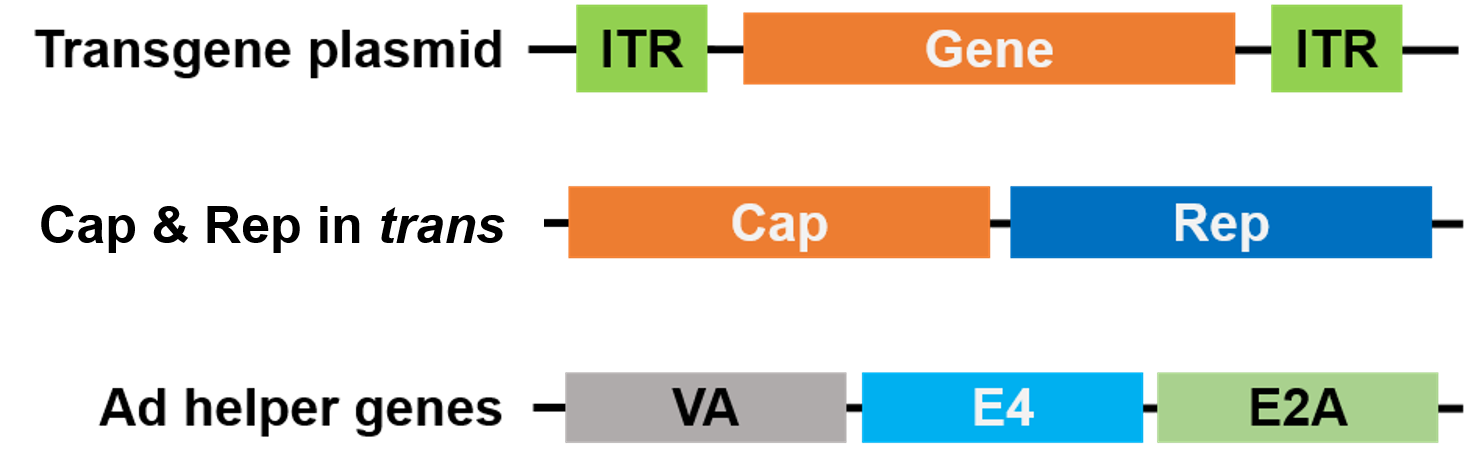
| Figure 3. Helper virus-free AAV. 3 plasmids are used for packaging. The gene of interest plasmid (top) contains the inverted terminal repeats (ITRs). A 2nd plasmid (middle) carries the AAV Cap and Rep genes, and the 3rd plasmid (bottom) carries the required genes from Adenovirus (Ad) |
Further, AAV exists in different serotypes, which affect the tissue specificity of AAV infection. Multiple serotypes enable researchers to either a) infect a broad range of host cells; or b) limit infection to one or a few tissues. GeneCopoeia’s AAVPrime™ AAV particles are available in multiple serotypes (Table 2).
| Serotype | Primary target tissue | Description |
| AAV-1 | Muscle | Best for cardiac muscle, skeletal muscle, neuronal and glial tissue. |
| AAV-2 | Muscle, Liver, Retina | Most commonly-used serotype. Best for neurons, muscle, liver, and brain. |
| AAV-3 | Megakaryocytes | Best for megakaryocytes, muscle, liver, lung, and retina. |
| AAV-4 | Retina | Best for neurons, muscle, brain, and retina. |
| AAV-5 | Lung | Best for lung, neurons, synovial joint, retina, and pancreas. |
| AAV-6 | Muscle, Lung | Best for lung, liver, and heart. |
| AAV-7 | Muscle, Retina, Neurons | Best for muscle, neurons, and liver. |
| AAV-8 | Liver | Best for muscle, brain, liver, and retina. |
| AAV-9 | Various | Best for muscle, heart, liver, lung, and brain. |
| AAV-10 | Pleura, CNS | Cloned from Cynomolgus, almost identical with AAVrh10 except for 12 amino acids in VP1. Best for lung, muscle, heart, NCS and liver. |
| AAV-DJ | Various | A mixture of 8 naturally-occurring serotypes. Efficiently transduces a wide variety of cell types in vitro. |
| AAV-DJ/8 | Various | A variant of AAV-DJ with a heparin binding domain (HBD) mutation, which permits infection of liver as well as other tissues in vivo. |
- High titers. Titer of purified particles can be up to 10^14 GC/ml (genome copies/ml)
- Versatile. Multiple serotypes enable usage in either a broad or limited range of host cells
- Low toxicity. Does not integrate into the host genome.
- Low immunogenicity. Minimal host immune response.
- Safe. Not associated with any human disease
AAVPrime™ particles are available for human and mouse ORFs up to 3 kb, in multiple serotypes and promoter options, in either standard purity (for in vitro use only) or purified (for in vivo use). Customers can order plasmid clones, custom-generated particles for genes of interest, or choose from pre-made options expressing genes such as fluorescent reporters (Figure 4).
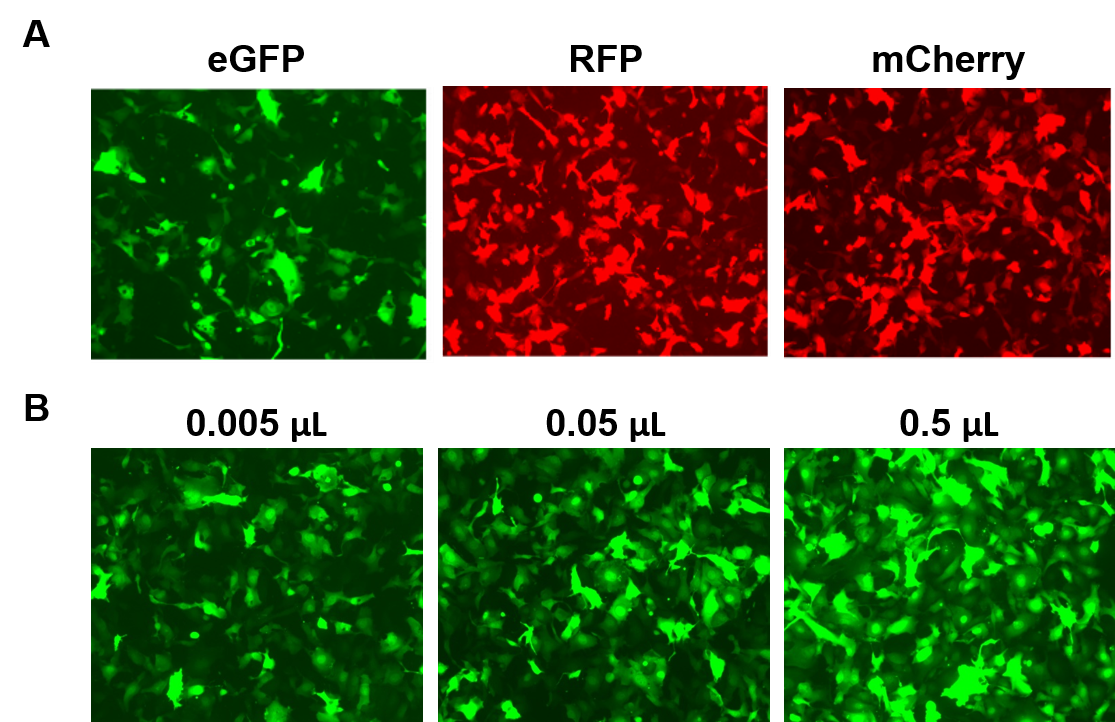
| Figure 4. Performance of GeneCopoeia’s AAVPrime™ particles. A. HT1080 cells in 24-well plates transduced with 0.5 µL of standard purity AAV expressing enhanced GFP (eGFP), red fluorescent protein (RFP), and monomeric Cherry (mCherry). B. HT1080 cells in 24-well plates were transduced with varying amounts of purified AAV expressing eGFP. Cells were visualized with a fluorescence microscope (Exposure time: 400 ms). |
Which should I choose: Lentivirus or AAV?
Choosing between lentivirus and AAV depends on many factors. For example, the fact that lentiviruses integrate into the genome can be both an advantage and a disadvantage, depending on what your needs are. Refer to the table below to help you decide:
| Larger inserts | Stable integration | No integration | Cell/tissue specificity | in vivo safety | |
| Lentivirus | ✔︎ | ✔︎ | |||
| AAV | ✔︎ | ✔︎ | ✔︎ |
Conclusions
At GeneCopoeia, we strive to provide you with the highest quality, cutting edge-technology in products for Functional Genomics and Cell Biology. We offer a wide gamut of products from genome-wide sets of plasmid DNA clones for ORFs, gene promoters, miRNAs, shRNA, and CRISPR, to powerful kits and reagents, to products for fluorescent cell structure probes, nucleic acid quantitation, and labeled antibodies. To learn more, please visit our website, www.genecopoeia.com, or contact us at inquiry@genecopoeia.com.
References
DeKelver, et al. (2010). Functional genomics, proteomics, and regulatory DNA analysis in isogenic settings using zinc finger nuclease-driven transgenesis into a safe harbor locus in the human genome. Genome Res 20, 1133. Dull, et al. (1998). A Third-Generation Lentivirus Vector with a Conditional Packaging System. J. Virol. 72, 8463. Ran, et al. (2015). In vivo genome editing using Staphylococcus aureus Cas9. Nature 526, 186. Sakuma, et al. (2012). Lentiviral vectors: basic to translational. Biochem. J. 443, 603. Samulski and Muzyczka (2014). AAV-Mediated Gene Therapy for Research and Therapeutic Purposes. Annu. Rev. Virol. 1, 427.| Copyright ©2017 GeneCopoeia, Inc. Email: inquiry@genecopoeia.com Tel: +1 (866) 360-9531 TNLV2-030817 |