– To validate ORF cDNA expression, shRNA knockdown, miRNA target or promoter activity
GeneCopoeia provides cell-based validation services to help researchers to validate the function and performance of expression clones using standard cell lines or customer’s own cell lines.
No matter you are studying transgene overexpression (ORF cDNA clones), gene knockdown (shRNA clones), transcription regulation in the promoter region (promoter reporter clones), or translation regulation by microRNA (microRNA target clones), GeneCopoeia’s expression clone collections and cell-based validation services are powerful tools to support your functional genomic research.
GeneCopoeia’s cell-based validation services cover ORF cDNA clone expression validation, shRNA knockdown validation, miRNA target validation and promoter activity validation.
Advantages |
|
GeneCopoeia’s cell-based validation services include:
ORF cDNA clone expression validation |
shRNA knockdown validation |
miRNA target validation |
Promoter activity validation |
|---|---|---|---|
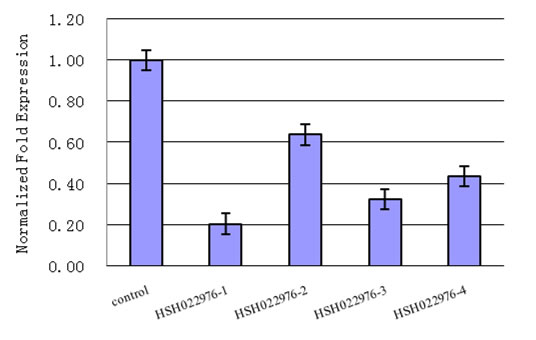
| Detect recombinant protein expression in mammalian cells or bacteria using western blot. | Measure the efficiency of shRNA knockdown of GOI on the messenger-RNA level using qRT-PCR. |
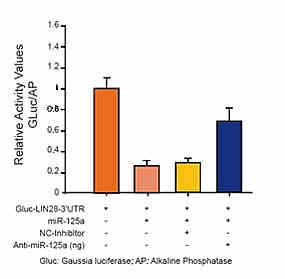
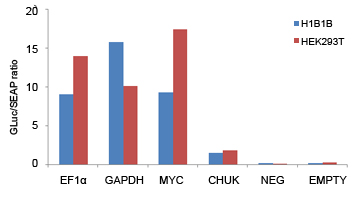
Validate the inhibitory effect of a particular miRNA on the 3’UTR region of GOI using luciferase reporter assays. | Detect exogenous promoter activation in mammalian cells using luciferase reporter assays. |
Largest clone collections to best suit your needs |
|||
miTarget™ 3’UTR miRNA Target Clones |
|||
Examples
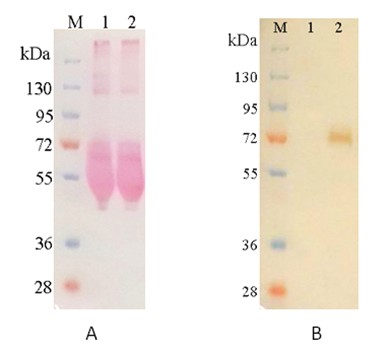
Method: Western Blot
| Figure 1. Secreted TPP1 recombinant protein expression in 293 cells.TPP1 protein was secreted into the culture medium 48hrs after transient transfection with TPP1 expression clone (Z5873). The culture media (15uL per lane) were collected, resolved using10% SDS-PAGE/Tris-Glycine, transferred to a NC membrane, and probed with the anti-Flag antibody (CGAB-DDK-0050). A) The NC membrane was stained with Ponceau S. B) The western blot results were visualized with a chromogenic substrate. The culture medium of HEK293T cells without transfection was used as the negative control (lane 1). |
Request a Quote
To request a quote for a cell-based validation service, please fill out the inquiry form here.
Related Services
Delivering high titer lentivirus in either purified form.
Stable cell line development services
For stable cell-line development, gene amplification, single clone selection, cell line characterization and more.